Updates on in situ generated active substances and products
During the 103rd CA meeting, this March, the long-awaited proposal on how to implement the general data requirements for in situ generated active substances (isAS) and biocidal products was published.
A very informative presentation prepared by ECHA, provided proposals on how to answer important regulatory questions such as:
- What is approved in the AS approval process of an isAS?
- Can the composition of the in situ generated active substance (isAS), approved in the AS stage, differ from the composition of the isAS applied for in product authorisation?
- Is technical equivalence needed for isAS?
Before answering those questions, we would like to provide some facts about the in situ generation (ISG).
ISG means the reaction of one or more precursor(s) to generate an active substance at the place of use for direct application without isolation, purification, storage or transport.
isAS definition follows the general substance definition in Articles 3(1)(c) of the Biocidal Product Regulation (EU) 528/2012 (BPR) and 3(1) of Regulation (EC) 1907/2006 (REACH) and including e.g. any impurity deriving from the in situ generation process used, such as reaction by-product, unreacted precursors, etc. are part of the isAS.
Figure 1 In situ generation reaction scheme [1]
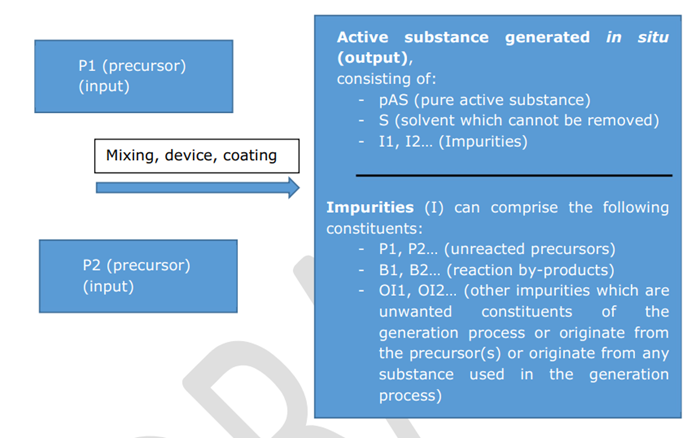
The whole in situ generation system (ISG) e.g. precursors, isAS and its impurities, device settings or catalysts needs to be assessed during an isAS approval.
There are different in situ systems. Currently 4 “case types” are identified and described in CA-July19-Doc.4.1. For case types 1-3, a precursor is placed on the market, which represents the biocide product. In case a non-marketable precursor (e.g. air, ambient water) is used for the in situ generation of the active substance (case type 4) the isAS is the biocidal product (isAS = BP).
The biocidal product (precursor) exists before the active substance (AS) and for the AS approval its composition is usually based on “example product”.
- What is approved in an isAS approval?
- Reference Specification will be set for precursors (not for the active substance as it is done in non in situ systems) marketed for biocidal purposes, using the results of the 5 batch analysis.
- Precursors not marketed for biocidal purposes (commodity chemicals) will have Standard Specification.
- No setting of reference specification or standard for not marketable precursors like air or ambient water
- Composition of the isAS should be provided in the form of a global composition.
Qualitative and quantitative ranges are due to variations of precursors amounts and device settings.
- Analytical data have to be provided to confirm the proposed global composition
- The concentration of the AS and impurities batches, tested during eco- and toxicological studies, should cover the global composition
Global composition can be adjusted by eco- and toxicological evaluation
2. Is technical equivalence (TE) needed for in situ generated active substances (isAS)?
- Not for the isAS
- Yes, for the precursor(s) marketed for biocidal purposes
- Full compliance of the precursor(s) with the set standards for commodity chemicals, in the isAS approval, must be demonstrated
3. Can the composition of the isAS, approved in the AS stage, differ from the composition of the isAS applied for in product authorisation?
- Yes, but any new data on isAS in product authorisation have to be assessed.
- At the active substance approval stage, an “example product”, which is generated based on set parameters of the device, will be assessed.
How will the stakeholders be informed about the isAS specification (global composition)?
- The composition of the isAS, including relevant impurities, will be published in the assessment report (AR), as usual. The rest of the composition will be published unless eCA accepts it is claimed as confidential.
- The implementation regulation (IR) includes the minimum purity of the precursor(s) and more information on the isAS (if applicable).
- Discussions between the product authorisation applicant and the applicant of the AS approval.
Conclusions:
What is to be done at the active substance approval stage?
For the precursors:
- Setting of standards (e.g. max allowed levels of heavy metals, EN norms to be followed) or reference specifications
- Minimum degree of purity – published in Implementation Regulation (IR)
- Assessment of precursors
For the isAS
- Assessment of the isAS
- Assessment of impurities (including left over precursors)
For the example product
- Assessment of the IGS – setting the parameters of the device
What must be done before the product authorisation by applicants?
The applicants need to check if a technical equivalence (TE) application is needed for their precursor(s). Furthermore, applicants must check whether the isAS they produce via IGS meets the specification of the approved isAS.
More guidance will be developed by ECHA on which criteria must be fulfilled for this comparison.
If the isAS generated by IGS meets the specification of the isAS approved, the applicant can use the approved isAS data package with a Letter of Access (LoA).
If not, additional data for the new isAS have to be provided at product authorisation stage.
The regulatory responses (proposals) in the published presentation, now provide clearer guidance for companies marketing in situ systems and for authorities.