in silico models

In silico models in the regulatory context
Computational non-testing methods (also known as in silico models) represent a fast and reliable alternative approach to in vivo and in vitro methods. They include, but are not limited to, 'Read-across' (automated tools for data gap filling or for analogue search), '(Quantitative) Structure-Activity Relationships ((Q)SAR)' models (e.g. statistical-based) and 'Expert systems' (built based on expert knowledge, including Mode of Action information).
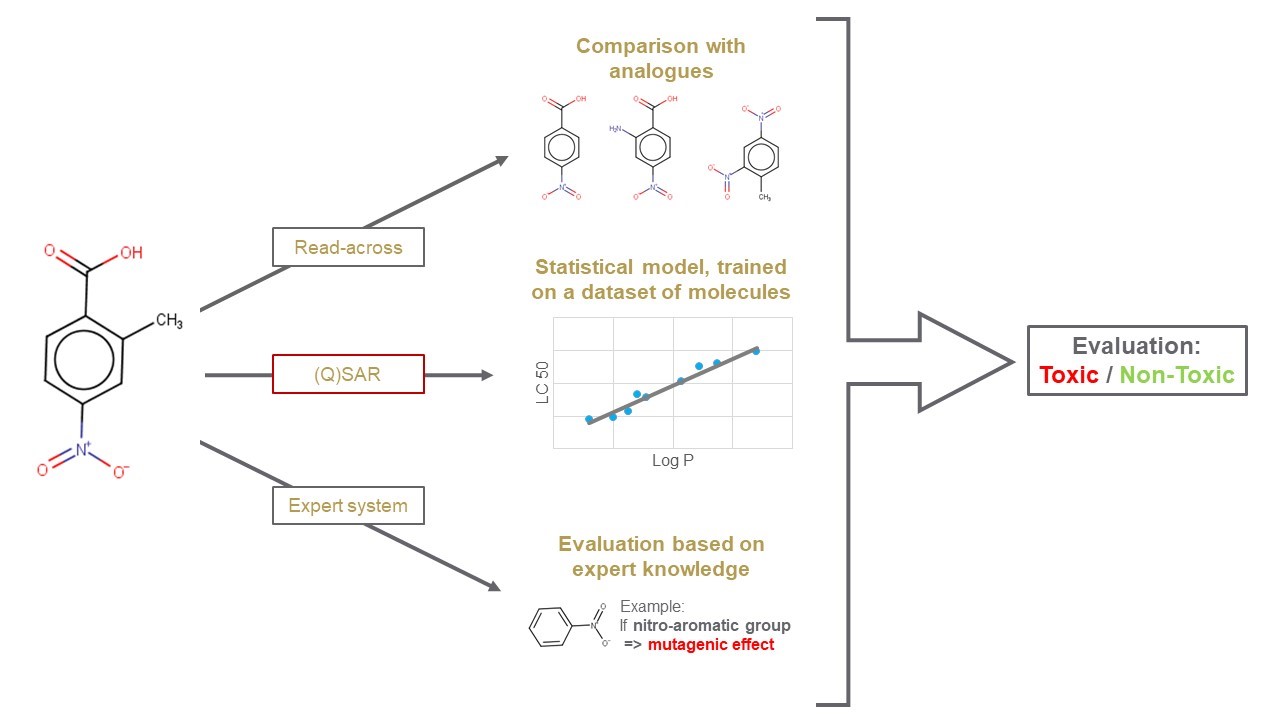
Why use in silico methods and models?
Data generated with in silico models can be used in the context of a number of regulatory frameworks in the EU and worldwide (e.g. Chemicals, Pharmaceuticals, Biocides, Cosmetics, Food Contact Materials, Medical Devices and Crop Protection).
For example the following tasks and questions can be supported by data derived from these models:
- Early screening can deliver results to support your decision making processes at an early stage of R&D of new substances.
- Obtained information can supplement experimental data in weight of evidence approaches.
- In order to meet the 3Rs principle: Replacement, Reduction and Refinement of animal testing.
- Testing of all metabolites (and impurities) is practically impossible e.g. due to timeline constraints and/or technical limitations
What We can do for you
Years of experience in the various regulatory areas covering the different categories of chemical substances allowed knoell to establish a team specifically dedicated to developments and use of in silico methods. Our team of experts in computational chemistry, ecotoxicology and human health can support you with
- Meeting the 3Rs principle: Replacement, Reduction and Refinement of animal testing
- Gathering information for environmental or human health safety assessments using multiple models covering different types of information (statistical-based models, knowledge-based models)
- Comparison of effects (ecotoxicological and toxicological properties) between two or more molecules (e.g. parent and impurities/metabolites, target and analogues, etc.)
- Grouping of chemicals for category formation and identification of candidate analogue(s) for read-across evaluation
- Evaluation of properties using multiple models covering different type of information (statistical-based models, knowledge-based models)
- Comparison of effects (ecotoxicological and toxicological properties) between two or more molecules
- Screening of chemical libraries for prioritisation: identification of less hazardous (e.g. for R&D) or more hazardous (e.g. for testing proposal) molecules
- Expert evaluation of results
Don't hesitate to get in touch with us to discuss your project in detail.
INTRODUCTION TO IN SILICO METHODS
From statistical-based model to complex knowledge-based system and read-across methods, in silico approaches represent a family of non-testing methods that can evaluate the same property or effect from different perspectives.
The main idea behind these approaches is the so-called 'similarity principle': structurally similar compounds should have similar biological activities. Based on this principle, properties and effects of a target chemical can be estimated by comparison with similar molecules with known experimental data. The three main families of in silico approaches are:
| (Q)SARs | Read-Across | Expert Systems |
|---|---|---|
 |
 |
 |
|
(Quantitative) Structure-Activity Relationships ((Q)SAR) models are theoretical statistical models that can be used to predict properties in a qualitative or quantitative manner. |
Grouping approaches are used for data-gap filling. Endpoint information from one chemical is used to predict the same endpoint for another structurally similar chemical. The OECD QSAR Toolbox is an example of a software that can be used for grouping and read-across. |
Expert systems make use of rules compiled by human experts and stored in a knowledge base. Types of expert systems include rule-based systems or statistic-based systems, as well as a combination of both schemes. |
How reliable are the results? The concept of (Q)SAR model validation
Despite differences in the uses and goals, the principle of “validity” of the model always plays a key role. The current internationally accepted definition of validity of (Q)SAR models was established in 2004 by The Organisation for Economic Co-operation and Development (OECD), which developed the “OECD principles for the Validation, for regulatory purposes, of (Quantitative) Structure-Activity Relationship models”. According to this document...
"To facilitate the consideration of a (Q)SAR model for regulatory purposes, it should be associated with the following information:
1) a defined endpoint
2) an unambiguous algorithm
3) a defined domain of application
4) appropriate measures of goodness-of-fit, robustness and predictivity
5) a mechanistic interpretation, if possible"
These principles define the validity of the model, not that of the obtained results, which must be defined within each regulatory areas, depending on the foreseen uses.
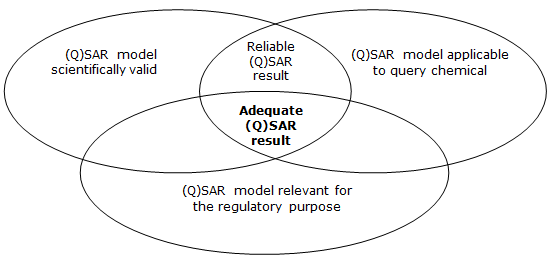
ECHA (2016): Practical guide - How to use and report (Q)SARs
IN SILICO MODELS WITHIN CURRENT REGULATORY FRAMEWORKS
In silico evaluations (also called “predictions”) of physico-chemical and environmental fate properties, as well as of (eco)toxicological effects represent fast, reliable and cost-effective solutions to obtain information useful for the registration of chemical substances.
The use of in silico models such as (Q)SARs is increasingly foreseen within several regulatory frameworks. The intended uses of these models differ among the areas of application (REACH, Biocides, Plant Protection Products, etc.); therefore, different guidelines have been developed or are currently in development.
Regulation (EC) No 528/2012
In silico methods are increasingly used within BPR. Examples of application of (Q)SAR and/or read-across approaches are
- Toxicological properties evaluation
- ED assessment
- Technical equivalence
- Metabolite(s) prediction/assessment
Regulation (EC) No. 10/2011
Possible application of in silico models (e.g. QSAR and read-across) are
- Assessment of non-intentionally added substances (NIAS)
- Impurities assessment
ICH Guideline M7
With Pharmaceuticals and Medical Devices, in silico methods can be used for
- Impurity assessment
Regulation (EC) No 1107/2009
In silico methods are being increasingly used under Regulation 1107/2009. Typical applications are
- Technical equivalence
- Residue definition for dietary risk assessment
- Metabolite(s) prediction/assessment
- ED assessment
REACH Regulation (EC) No 1907/2006; New substances: TSCA Section 5
Results from (Q)SAR model predictions can be used for
- REACH dossier creation
- TSCA submissions under Section 5 (pre-manufacture notification)
Regulation (EC) No 1223/2009
In silico methods can be used for
- Estimation of the toxicological profile of substances
- Grouping strategies
Case Study - LIFE CONCERT REACH project
Life Concert Reach is an european network that offers easy access to and use of the most important databases for non-testing methods (NTMs). The new platform for in silico models improves the usability and acceptance of results from NTMs (such as (Q)SAR models and read-across) for the registration of chemical substances under various regulatory frameworks.
Starting in 2018, knoell experts collaborated with scientists from the Technical University of Denmark, the Institutto di Ricerche Farmacologiche Mario Negri IRCCS, BIGCHEM, Kode s.r.l. and SC Sviluppo chimica to finally provide simple access to NTMs and to improve their acceptance for legitimacy in the process.
Visit the offical LIFE CONCERT REACH website.




